Which Is a Component of John Dalton's Atomic Theory
B Atoms of the same element have the same properties. Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks.

Pin On Science House Resources
The atoms of different elements are the same.

. Instead of being units that are made up of great mass atomic theory experiments were able to prove that a vast majority of atoms are basically just empty space. Which is a component of John Daltons atomic theory. Terms in this set 7 Part 1.
Blackline Master 66-1 - Worksheet 21 Terms. Daltons theory stated hat all atoms of a given element have exactly the same size and mass. It entails the adhering to proposes.
Asolution contains 225 g of sodium chloride nacl dissolved in enough water to make a 025 l. Daltons atomic theory can be summarized in 5 key points. Development of Atomic Theory Democritus Aristotle and John Dalton 20 Terms.
When a nucleus absorbs a neutron and then breaks apart there are many products of the reaction. Dalton found an atomic theory of matter could elegantly explain this common pattern in chemistry in the case of Prousts tin oxides one tin atom will combine with either one or two oxygen atoms. 2500 g Solution 150 g NaCl100 g Solution 375 g NaCl.
Which is a component of John Daltons atomic theory. 39 Although all parts postulates of Daltons atomic theory are important which one of the postulates is crucial to explain the observations summarized by the Law of Definite Proportions. The ratio of atoms in a compound is fixed.
What was the main reason behind the failure of Daltons atomic theory. Daltons Atomic Theory the laws its built on and the law he derived from it. 1 Components include indivisible little fragments atoms 2 All atoms of the exact same component equal.
A reaction can create or destroy atoms as well as rearrange them. Mixture is uniform throughout sample is the same. A concept of chemical mix initial specified by John Dalton in 1803.
YOU MIGHT ALSO LIKE. The primary reason behind the failure of Daltons atomic theory was the claim that atoms are indivisible. While all atoms of an element were identical different elements had atoms of differing size and mass.
3 Show answers Another question on Chemistry. Chemistry 22062019 0910 cheesedoodle. 135 and 27 form a ratio of 12.
Atoms of different elements vary in size and mass. Combination of substances in which each component retains its own physical properties. What is not a product of a nuclear fission reaction.
A Matter is composed of atoms. Dalton showed that matter always combined in fixed ratios based on mass or based on volume in the case of gases. Daltons atomic theory was a logical hypothesis on the idea of the issue and it expressed that all matter was comprised of little unified particles known as atoms.
John Daltons Atomic Theory. The modern proof for the atomic nature of matter was first proposed by the English chemist John Dalton. Dalton noted from these percentages that 100g of tin will combine either with 135g or 27g of oxygen.
Each element is composed of atoms. SNC1D - Development of the Atomic Theory. What is component of John Daltons atomic theory.
All atoms of the same element are identical. Terms in this set 28 John Dalton attempted to do what. Calculate how many grams of NaCl are in 2500 g of solution.
Which is a component of John Daltons atomic theory. Atoms are tiny indivisible and indestructible particles of an element. This hypothesis recommended that all these iotas atoms of a component were indistinguishable various components had molecules of contrasting size and mass.
What is component of John Daltons atomic theory. Atoms of different elements have different properties. Mass of the solution.
Which is a component of John Daltons atomic theory. 3 Atoms can neither be produced neither ruined. Atoms of an element are uniform in size and mass.
Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. An atom is a small particle of matter that can be broken down. Daltons atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios.
1 Get Other questions on the subject. By declaring atoms indivisible Dalton meant that there was no component inside atoms and it could be broken down no further. Daltons atomic theory states that chemical reactions involve combination separation or rearrangement of atoms.
THIS IS THE BEST ANSWER The material is composed of the smallest indivisible particle named as an atom. In 1803 John Dalton published the first comprehensive atomic theory in A New System of Chemical Philosophy. His theory shows that the early Greeks held the correct concept that matter consists of a combination of basic elements.
Compounds are formulated by a fixed ratio of individual atoms of elements. He proposed that the elements are. Atoms are actually made of positive components called protons negative components called electrons and neutral components that are called neutrons.
Various aspects have various sorts of atom. While all atoms of an element were identical different elements had atoms of differing size and mass. The ratio of atoms in a compound is fixed.
The concentration of NaCl is 150 by mass that is there are 150 g of NaCl every 100 g of solution.

Class 9 Science Notes Chapter 4 Structure Of The Atom Science Notes Chemistry Classroom Biology Notes

Worksheet History Of The Atomic Model Matter Science High School Chemistry Atom

The Advent Of Modern Atomic Theory Vocabulary 1 Theory 2 Democritus 3 Science 4 John Dalton 5 Dalton S Model 6 Dalton S Atomic Theory 7 Law Of Ppt Download

Who Is Considered The Pioneer Of Modern Atomic Theory Atom Atomic Theory Dalton Atomic Model

Daltons Atomic Theory Easy Science Atomic Theory John Dalton Atomic Theory Chemical Changes

Dalton S Atomic Theory Atomic Theory Atom Model John Dalton Atomic Theory

What Evidence Did Dalton Have That Atoms Exist Quora

Atomic Theory Why Do We Believe In Atoms Ppt Download

John Dalton And Atomic Theory Introduction To Chemistry

Dalton S Atomic Theory Overview Modern Application Expii

John Daltons Atom Theory Model Did Include The Charges Dalton Atomic Model Atomic Theory Atom Dalton
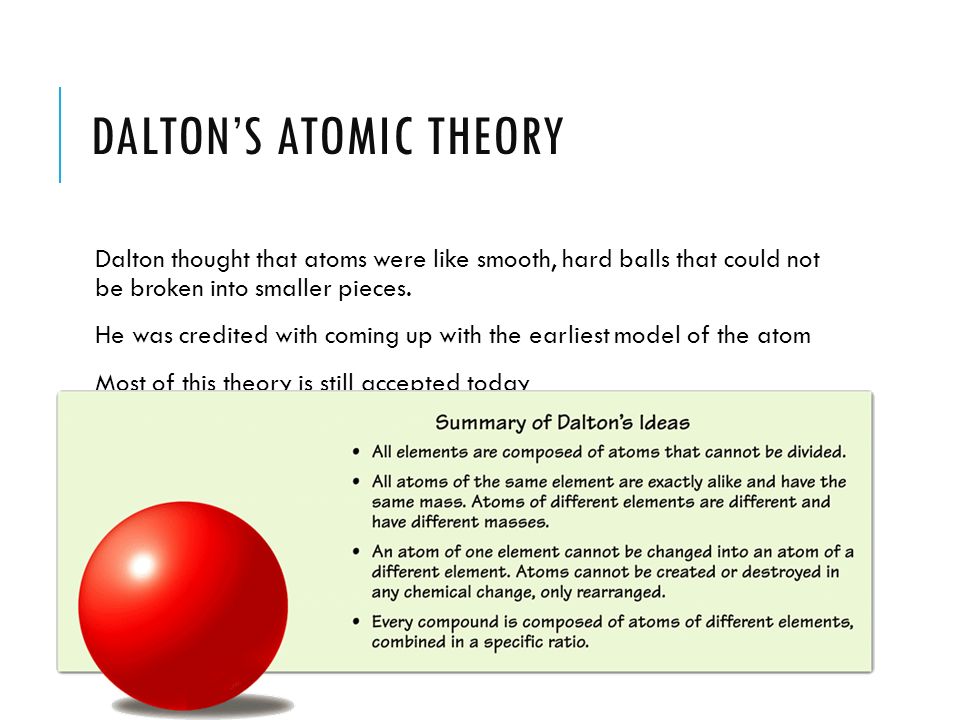
Notes 4 1 Intro To Atoms Ppt Video Online Download

Daltons Atomic Theory Dalton Stated That Elements Consisted Of Tiny Particles Called Atoms He Also Called The Elements Pure Substances Because All Atoms Ppt Download

Scientific Explorer May 2012 Dalton Atomic Model Atom Model Dalton Model

John Dalton Biography Discoveries Atomic Model Facts Britannica
How Were The Different Atomic Theories And Models Conceptualized If The Atom Could Not Be Seen Quora

Comments
Post a Comment